`=>` All the lanthanoids are silvery white soft metals and tarnish rapidly in air.
`=>` The hardness increases with increasing atomic number, samarium being steel hard.
`=>` Their melting points range between `1000` to `1200 K` but samarium melts at `1623 K`.
`=>` They have typical metallic structure and are good conductors of heat and electricity.
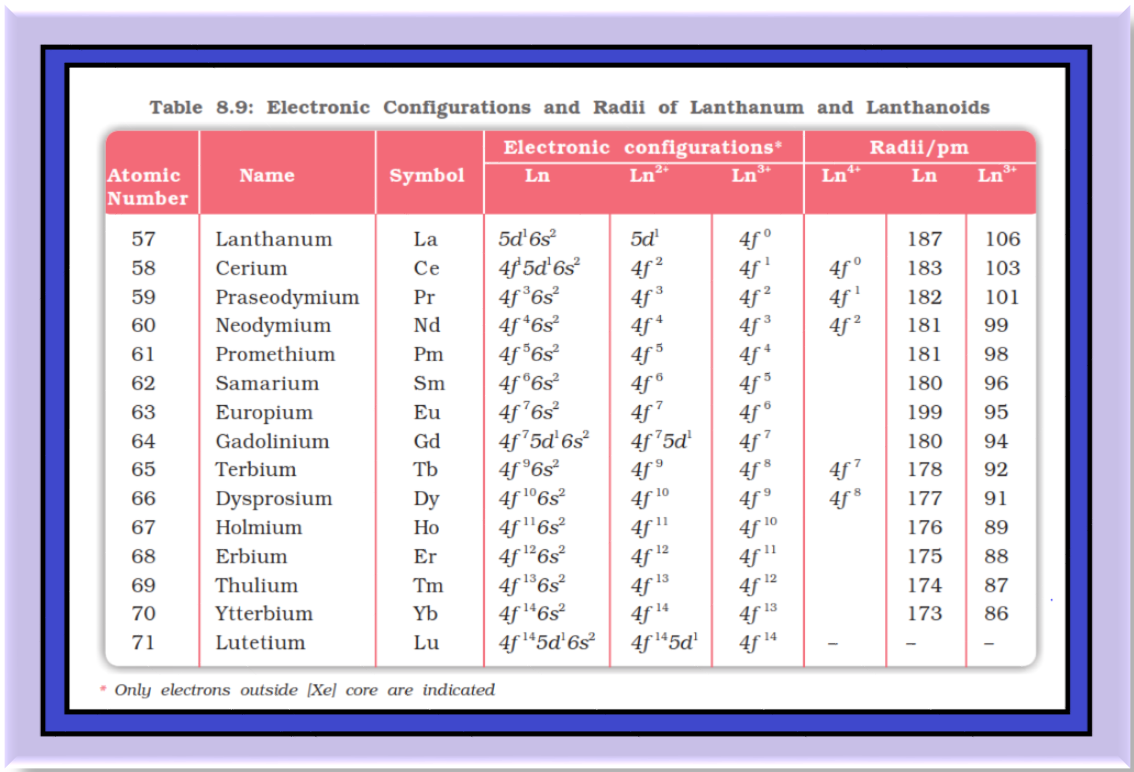
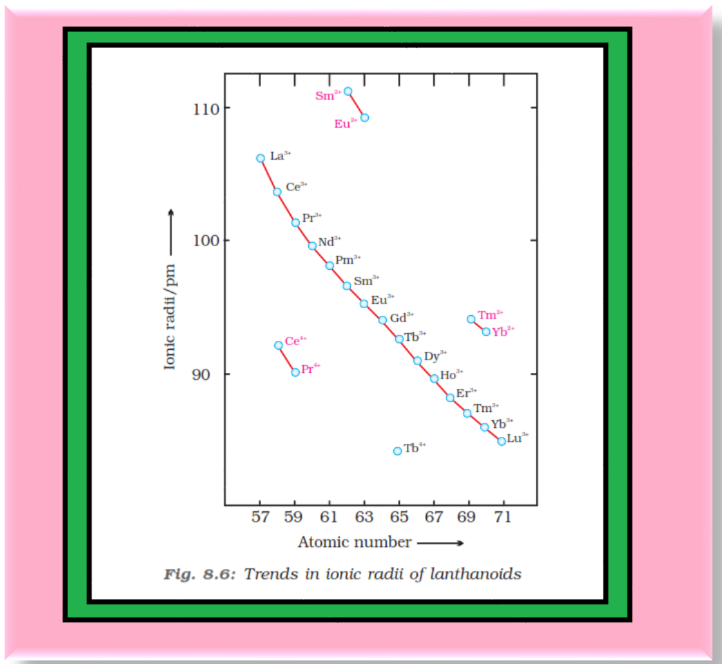
`=>` Density and other properties change smoothly except for `color{red}(Eu)` and `color{red}(Yb)` and occasionally for `color{red}(Sm)` and `color{red}(Tm)`.
`color{green}(text(Colour ))` : ● Many trivalent lanthanoid ions are coloured both in the solid state and in aqueous solutions.
● Colour of these ions may be attributed to the presence of `color{red}(f)` electrons.
● Neither `color{red}(La^(3+))` nor `color{red}(Lu^(3+))` ion shows any colour but the rest do so.
● However, absorption bands are narrow, probably because of the excitation within `f` level.
`color{green}(text(Magnetism ))` : ● The lanthanoid ions other than the `color{red}(f^0)` type `color{red}((La^(3+))` and `color{red}(Ce^(4+)))` and the `color{red}(f^( 14))` type `color{red}((Yb^(2+))` and `color{red}(Lu^(3+)))` are all paramagnetic.
● The paramagnetism rises to maximum in neodymium.
`color{green}(text(Ionisation Enthalpy ))` : ● The first ionisation enthalpies of the lanthanoids are around `600 kJ mol^(–1)`, the second about `1200 kJ mol^(–1)` comparable with those of calcium.
● The variation of the third ionisation enthalpies indicates that the exchange enthalpy considerations (as in `color{red}(3d)` orbitals of the first transition series), appear to impart a certain degree of stability to empty, half-filled and completely filled orbitals `f` level.
● This is indicated from the abnormally low value of the third ionisation enthalpy of lanthanum, gadolinium and lutetium.
`color{green}(text(Chemical Reactivity ))` : ● In their chemical behaviour, in general, the earlier members of the series are quite reactive similar to calcium but, with increasing atomic number, they behave more like aluminium.
● Values for `color{red}(E^⊖)` for the half-reaction :
`color{red}(L n^(3+) (aq) + 3e^(–) → L n(s))`
are in the range of `–2.2` to `–2.4 V` except for `Eu` for which the value is `– 2.0 V`.
● This is, of course, a small variation.
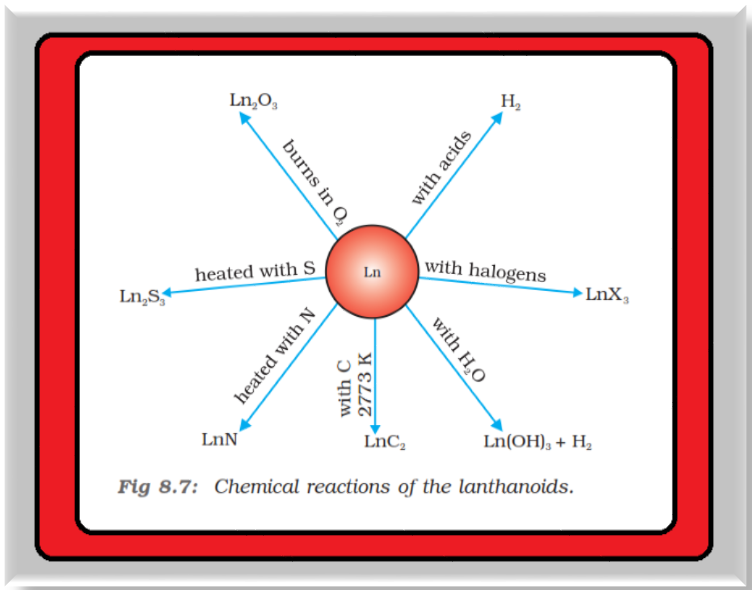
● The metals combine with hydrogen when gently heated in the gas.
● The carbides, `color{red}(L n_3C)`, `color{red}(L n_2C_3)` and `color{red}(L nC_2)` are formed when the metals are heated with carbon.
● They liberate hydrogen from dilute acids and burn in halogens to form halides.
● They form oxides `color{red}(M_2O_3)` and hydroxides `color{red}(M(OH)_3)`. The hydroxides are definite compounds, not just hydrated oxides. They are basic like alkaline earth metal oxides and hydroxides. Their general reactions are depicted in Fig. 8.7.
`color{green}(text(Uses ))` : ● The best single use of the lanthanoids is for the production of alloy steels for plates and pipes.
● A well known alloy is mischmetall which consists of a lanthanoid metal `(~ 95%)` and iron `(~ 5%)` and traces of `color{red}(S)`, `color{red}(C)`, `color{red}(Ca)` and `color{red}(Al)`.
● A good deal of mischmetall is used in `color{red}(Mg)`-based alloy to produce bullets, shell and lighter flint.
● Mixed oxides of lanthanoids are employed as catalysts in petroleum cracking.
● Some individual `color{red}(L n)` oxides are used as phosphors in television screens and similar fluorescing surfaces.
`=>` All the lanthanoids are silvery white soft metals and tarnish rapidly in air.
`=>` The hardness increases with increasing atomic number, samarium being steel hard.
`=>` Their melting points range between `1000` to `1200 K` but samarium melts at `1623 K`.
`=>` They have typical metallic structure and are good conductors of heat and electricity.
`=>` Density and other properties change smoothly except for `color{red}(Eu)` and `color{red}(Yb)` and occasionally for `color{red}(Sm)` and `color{red}(Tm)`.
`color{green}(text(Colour ))` : ● Many trivalent lanthanoid ions are coloured both in the solid state and in aqueous solutions.
● Colour of these ions may be attributed to the presence of `color{red}(f)` electrons.
● Neither `color{red}(La^(3+))` nor `color{red}(Lu^(3+))` ion shows any colour but the rest do so.
● However, absorption bands are narrow, probably because of the excitation within `f` level.
`color{green}(text(Magnetism ))` : ● The lanthanoid ions other than the `color{red}(f^0)` type `color{red}((La^(3+))` and `color{red}(Ce^(4+)))` and the `color{red}(f^( 14))` type `color{red}((Yb^(2+))` and `color{red}(Lu^(3+)))` are all paramagnetic.
● The paramagnetism rises to maximum in neodymium.
`color{green}(text(Ionisation Enthalpy ))` : ● The first ionisation enthalpies of the lanthanoids are around `600 kJ mol^(–1)`, the second about `1200 kJ mol^(–1)` comparable with those of calcium.
● The variation of the third ionisation enthalpies indicates that the exchange enthalpy considerations (as in `color{red}(3d)` orbitals of the first transition series), appear to impart a certain degree of stability to empty, half-filled and completely filled orbitals `f` level.
● This is indicated from the abnormally low value of the third ionisation enthalpy of lanthanum, gadolinium and lutetium.
`color{green}(text(Chemical Reactivity ))` : ● In their chemical behaviour, in general, the earlier members of the series are quite reactive similar to calcium but, with increasing atomic number, they behave more like aluminium.
● Values for `color{red}(E^⊖)` for the half-reaction :
`color{red}(L n^(3+) (aq) + 3e^(–) → L n(s))`
are in the range of `–2.2` to `–2.4 V` except for `Eu` for which the value is `– 2.0 V`.
● This is, of course, a small variation.
● The metals combine with hydrogen when gently heated in the gas.
● The carbides, `color{red}(L n_3C)`, `color{red}(L n_2C_3)` and `color{red}(L nC_2)` are formed when the metals are heated with carbon.
● They liberate hydrogen from dilute acids and burn in halogens to form halides.
● They form oxides `color{red}(M_2O_3)` and hydroxides `color{red}(M(OH)_3)`. The hydroxides are definite compounds, not just hydrated oxides. They are basic like alkaline earth metal oxides and hydroxides. Their general reactions are depicted in Fig. 8.7.
`color{green}(text(Uses ))` : ● The best single use of the lanthanoids is for the production of alloy steels for plates and pipes.
● A well known alloy is mischmetall which consists of a lanthanoid metal `(~ 95%)` and iron `(~ 5%)` and traces of `color{red}(S)`, `color{red}(C)`, `color{red}(Ca)` and `color{red}(Al)`.
● A good deal of mischmetall is used in `color{red}(Mg)`-based alloy to produce bullets, shell and lighter flint.
● Mixed oxides of lanthanoids are employed as catalysts in petroleum cracking.
● Some individual `color{red}(L n)` oxides are used as phosphors in television screens and similar fluorescing surfaces.